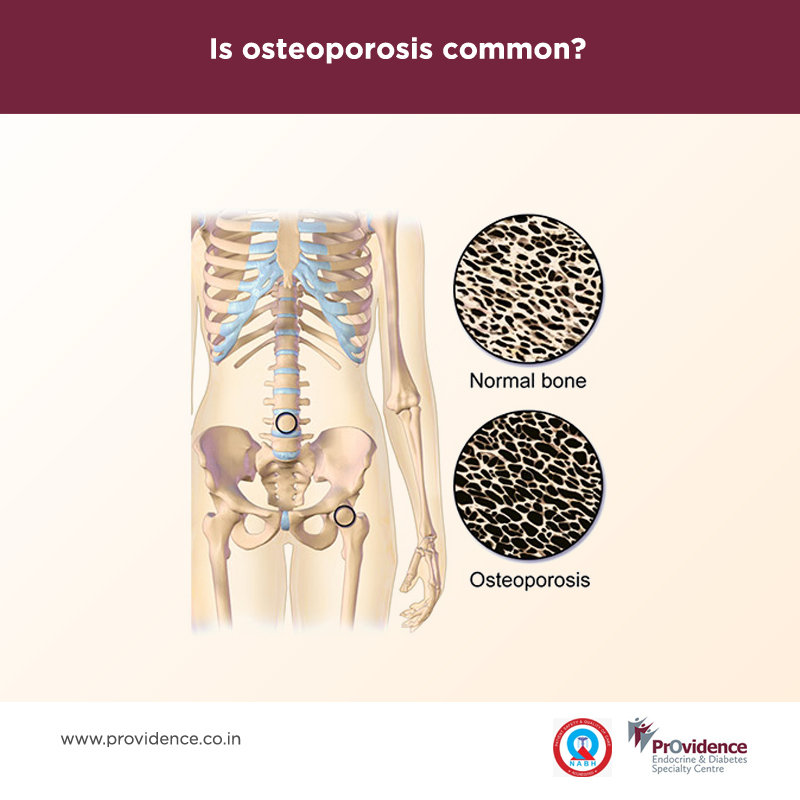
Calcium is required for all age groups to build and maintain strong bones. Calcium is essential for the heart, muscles and nerves to function properly. Calcium requirement varies with age and sex. Women younger than 50 years require 1000mg/day, 51 and above requires 1200mg /day. Men up to 70 years require 1000mg /day calcium and 71 and above requires 1200mg/day (National Osteoporosis Foundation). For proper absorption of calcium, our body needs vitamin D in the right amount. The daily requirement of Vitamin D for women and men under age 50 is 400-800IU/day, above age 50 and older require 800-1000 IU/day.
If our body fails to obtain adequate calcium from the food we eat, it is taken from our bones and gradually can lead to making our bones weak, porous and easier to break. A variety of foods contain calcium, if it is consumed in the right amount, it’s enough to meet daily calcium requirements. Common sources of calcium are milk & milk products (curd, cheese, yoghurt etc), Green Leafy Vegetables like drumstick leaves, spinach, kale, broccoli etc, fish with edible soft bone-like anchovy, sardines etc and nuts and seeds like almonds, pistachios and sesame seeds. Ragi is also a rich and cheap source of calcium. It is good to understand how much calcium is present in the food that you consume e.g 100 ml of milk contains 120 mg of calcium and 100 mg of ragi contains 340 mg of calcium. Caffeine present in coffee, tea and soft drinks may interfere and decrease calcium absorption, so use in moderation. Fish like tuna, mackerel, and sardines are a source of vitamin D also.
Author- Mrs. Rejitha